ISO 13485 : MEDICAL DEVICES MANAGEMENT SYSTEM
What is ISO 13485: Medical Devices Management System?
ISO 13485: Medical Devices Management System is the globally recognized standard for establishing and maintaining a quality management system for medical devices. It outlines specific quality system requirements for medical devices to ensure the design, production, installation, and servicing of medical equipment meet both customer needs and regulatory compliance.
Obtaining medical device certification is a critical step for any manufacturer looking to ensure compliance with international safety and quality standards.
This standard is tailored for the healthcare sector, especially companies involved in:
- Manufacturing and assembling iso 13485 medical devices
- Supplying parts or raw materials
- Sterilization services
- Technical support or calibration
Implementing a strong quality management system for medical devices is key to minimizing risks, improving traceability, and ensuring regulatory compliance.
By implementing the ISO 13485 quality management system, businesses demonstrate their ability to deliver safe and effective medical devices consistently, enhancing product reliability and patient safety.
Why ISO 13485 Certification is Crucial for Medical Device Companies
Earning an ISO 13485 certification is not optional for medical device manufacturers aiming to compete in regulated markets. It is a legal and commercial necessity in many regions, including the EU, USA, and the Middle East.
Key benefits of ISO 13485 include:
- Regulatory Compliance: Aligns with MDR, FDA, and SFDA requirements
- Risk Reduction: Prevents nonconforming product issues and recalls
- Customer Trust: Demonstrates accountability and competence
- International Access: Recognized by authorities and buyers worldwide
- Improved Processes: Encourages effective quality management
Organizations seeking 13485 certification enhance their reputation and operational control, especially when guided by experienced ISO 13485 certification companies.
A robust quality management system for medical devices is the foundation for risk reduction, regulatory compliance, and consistent product quality throughout the lifecycle.

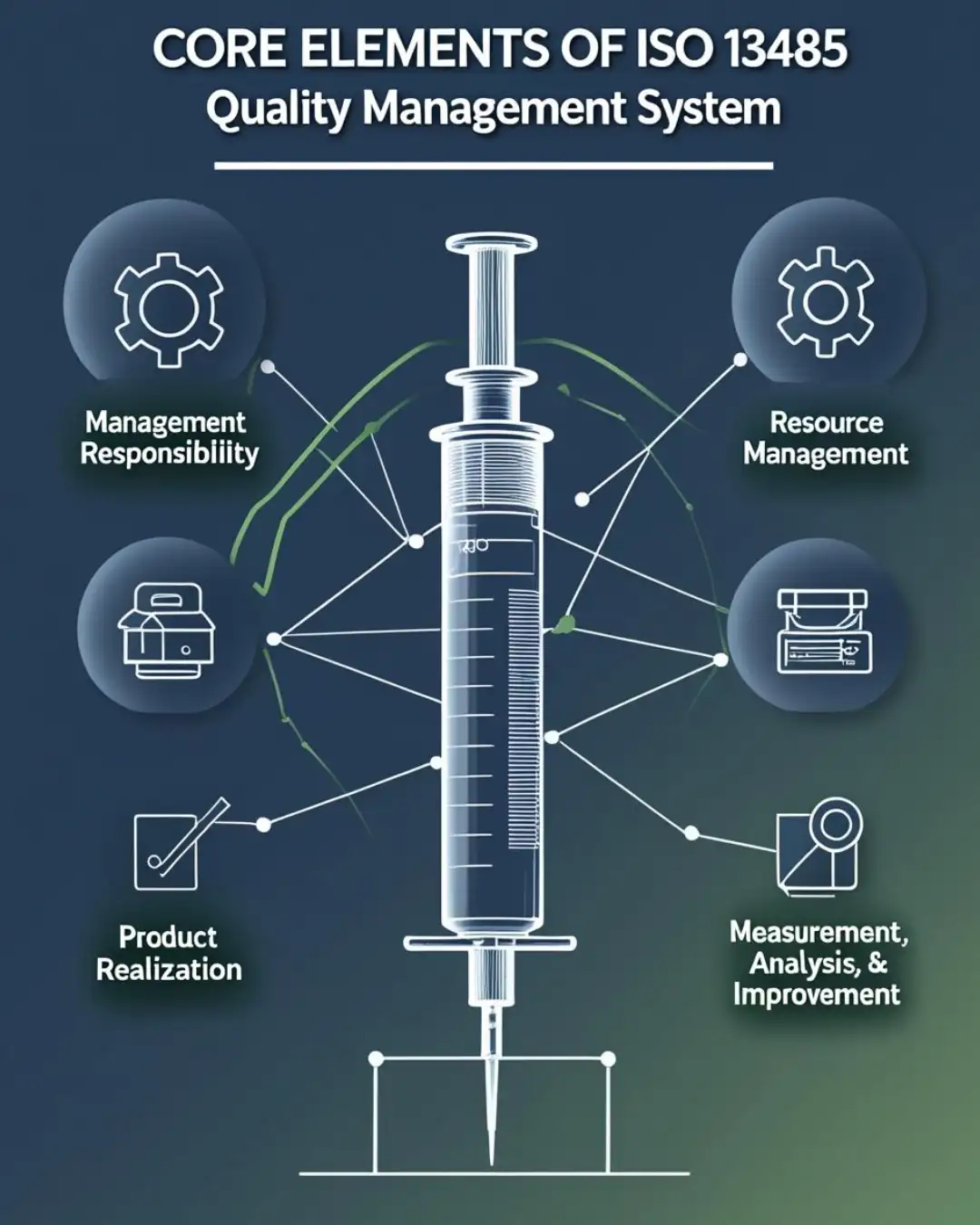
Core Elements of ISO 13485 Quality Management System
The ISO 13485 quality management system focuses on regulatory safety and product lifecycle control. Unlike general quality standards, it places specific emphasis on risk management and traceability within medical device production.
Key components of this system include:
- Design and development controls
- Production and process validation
- Sterile environment procedures
- Complaint handling and vigilance
- Documentation and record management
Each of these is a critical part of achieving and maintaining ISO 13485 certified status. Businesses must establish full compliance with quality system requirements for medical devices to remain competitive.
Who Needs ISO 13485 Certification?
The standard applies to a wide range of entities in the healthcare product ecosystem:
- Medical device manufacturers and OEMs
- Distributors and importers
- Calibration and testing laboratories
- Sterilization and packaging service providers
- Product designers and consultants
If your organization is in the supply chain of iso 13485 medical devices, achieving 13485 certification helps guarantee market access and regulatory approval. Even startups benefit by aligning early with iso 13485 quality management system practices.
Understanding and adhering to medical device regulations UK is essential for product approval and successful entry into the healthcare market. as Being recognized as an ISO 13485 certified company enhances your credibility and assures clients that your medical devices meet strict quality benchmarks.
ISO 13485 Certification Process: Step-by-Step
Achieving iso 13485 certification involves a structured approach:
- Gap Analysis – Assess existing processes against the quality system requirements for medical devices
- System Development – Design and document your iso 13485 quality management system
- Implementation – Train teams and roll out policies
- Internal Audit – Ensure system effectiveness
- Certification Audit – Performed by recognized iso 13485 certification companies
- Ongoing Compliance – Maintain iso 13485 certified status through regular reviews
This certification process is essential for any company aiming to establish itself as a reliable provider of iso 13485 medical devices.
With expert medical device consultancy We offer, your organization receives tailored guidance to meet regulatory and quality system requirements efficiently.

ISO CERT INTERNATIONAL – Your Partner for ISO 13485 Certification
Medical device consultancy by ISO CERT INTERNATIONAL provides end-to-end support, from regulatory alignment to achieving ISO 13485 compliance efficiently.
At ISO CERT INTERNATIONAL, we specialize in helping healthcare and life science companies achieve ISO 13485: Medical Devices Management System certification with confidence and clarity. We present:
- Expert guidance on all quality system requirements for medical devices
- Proven track record in helping businesses become iso 13485 certified
- Strategic support from system design to audit success
- Competitive pricing and efficient certification timelines
We work with startups, manufacturers, and global suppliers to build, optimize, and certify iso 13485 quality management systems that align with regulatory expectations and industry best practices.
Becoming an ISO 13485 certified company demonstrates your commitment to producing safe, reliable, and high-quality medical devices in compliance with global expectations.
If you are interested in implementing ISO 13485 in your organization, you can contact us for more information. We are a certified ISO 13485 consultant that can help you design, develop, implement, audit, and improve your QUALITY management system. We have the expertise, experience, and tools to help you achieve your goals.
